A New Cellular Frontier
What if you could find another way to fight cancer by approaching it from an engineering perspective, acting on the cellular process by which tissues harden into tumors? Or how about circumventing a host of heart diseases by preventing, perhaps even reversing, the actual stiffening of cardiovascular tissue?
First you’d have to understand how cells sense and respond to their mechanical environment within tissues and tumors, processes that remain largely unknown — at least for the moment — according to UC Santa Barbara mechanical engineering professor Otger Campàs. For his efforts in developing tools to explore the little-known territory of the mechanical aspect of cellular development Campàs has received a National Science Foundation (NSF) Faculty Early Career Development (CAREER) Award.
“It’s an honor,” said Campàs, who holds the UCSB Mellichamp Endowed Chair in Systems Biology and Bioengineering. “I know very talented young scientists and engineers who have gotten this award, so it’s a privilege to become part of that group.”
The five-year, $500,000 award for “decoding the mechanical control of tissue growth” will assist Campàs and his team in gaining a better understanding of how cells perceive and respond to mechanical forces around them. That knowledge could, in turn, be tied back to the biochemical and genetic information cells exchange with each other, for an even clearer and more in-depth picture of how organisms grow and develop.
“You have millions of cells in your body; how do they know how to build a specific structure in a three-dimensional space?” he said. “This is actually what we’re trying to understand.” It’s a process that can’t be observed in petri dishes and test tubes, because genetics and biochemistry aside, developing cells rely also on mechanical forces in their natural 3D environment — that is, surrounded by other cells and tissue structures — to “decide” what to develop into.
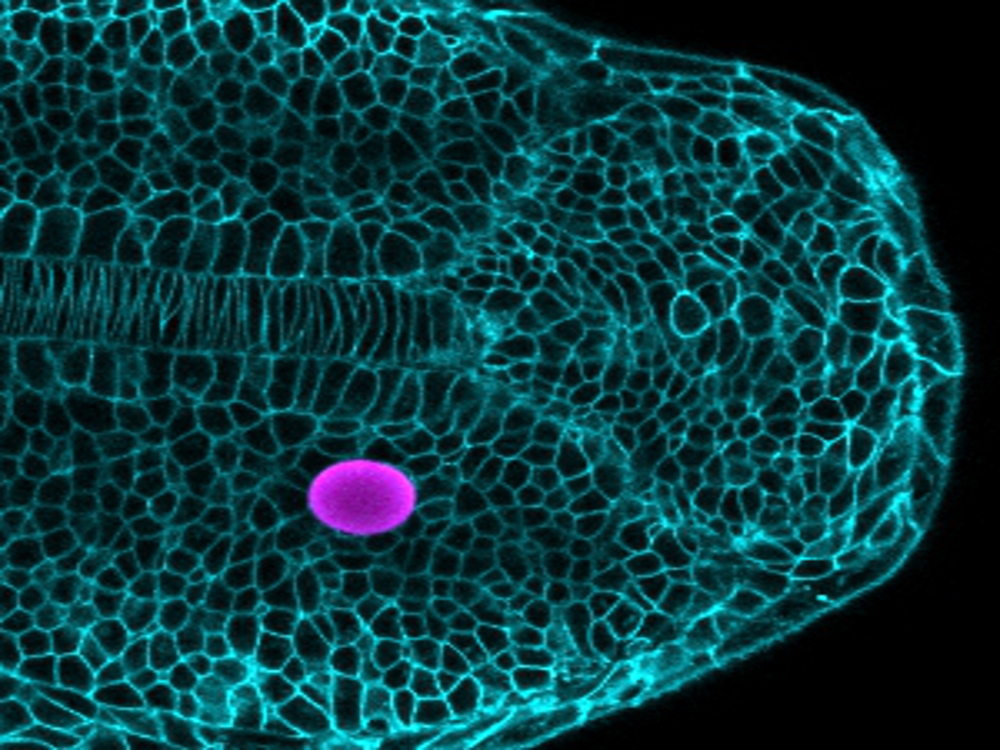
“Cells also use their ‘sense of touch’ to sculpt organs into functional structures,” Campàs explained. But there was no tool to measure the tiny forces developing cells in the organism exert or perceive that take them on their developmental pathways toward the various cell types they become.
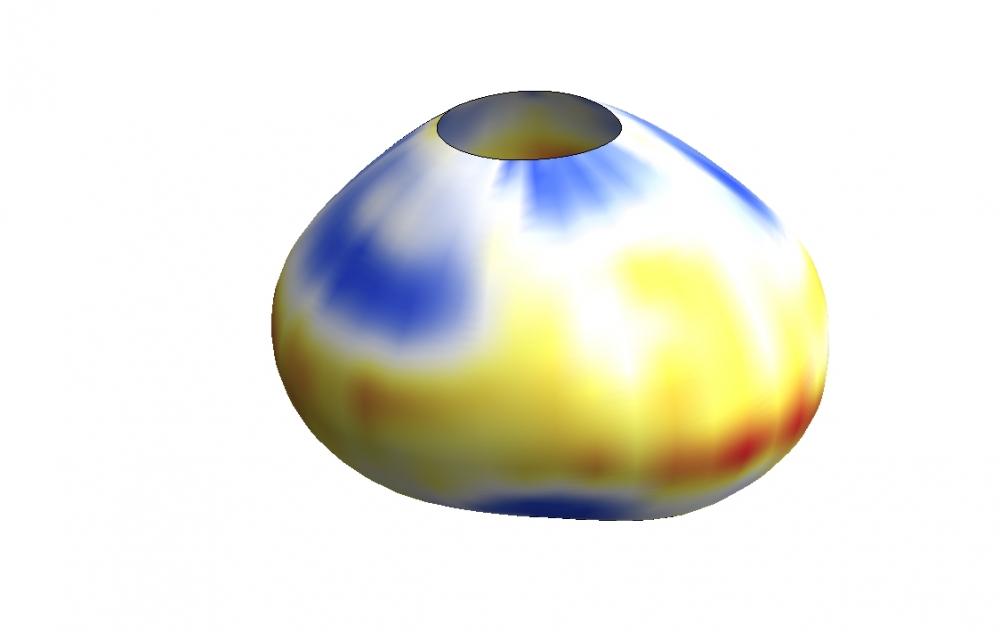
So he and his team made some. Using oil-based and also ferromagnetic microdroplets, the researchers devised ways to precisely apply and measure mechanical forces on cells in developing fish and mouse embryos, leading to the first cell-scale measurements of mechanics of tissue growth in living organisms.
“An organ is a ‘machine’ that performs specific functions,” Campàs said. All organs are three-dimensional structures whose functions are largely determined by their mechanical properties and their shapes, he explained. Finding out how the material sculpts itself into the machine will not only contribute to fundamental knowledge in biology, it could also provide valuable insight on anomalous conditions of cellular mechanics, such as the formation of tumors and fibroids, or the hardening of arteries and what mechanically causes those phenomena. This emerging perspective could eventually hasten or make more sophisticated treatments for the many and various diseases and symptoms associated with cellular mechanics.
The NSF’s Faculty Early Career Development (CAREER) program offers the organization’s most prestigious awards in support of junior faculty who exemplify the role of teacher-scholars through outstanding research, excellent education and the integration of education and research within the context of the mission of their organizations. Such activities are intended to build a firm foundation for a lifetime of leadership in integrating education and research.



