An Elegant Solution
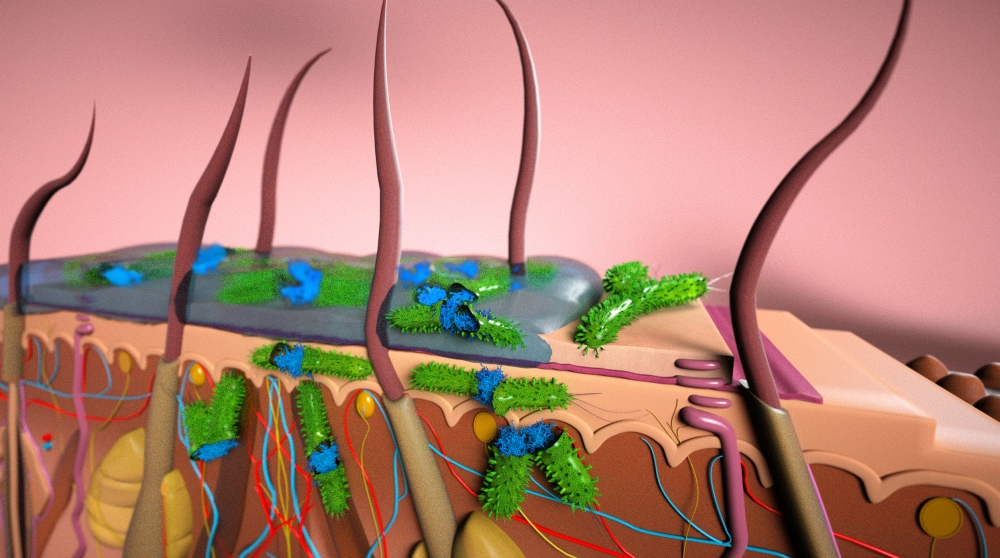
Microbial biofilms are the bane of chronic wound sufferers and wound care specialists around the world. Colonies of various microorganisms that are embedded in a protective matrix, microbial biofilms adhere to a surface — often the skin — and impair the healing process.
Affixed to each other by self-produced secretions of polysaccharides, proteins and other substances, and shielded by these secretions and the outermost layer of skin under which they often form, these microbes are able to resist antibiotics and continue to prey on the tissue beneath. The results are wounds that are slow to heal, or continue to reappear. It’s a circumstance both painful and expensive: In the United States last year, up to $75 billion was spent for the treatment of recurring and resistant wounds.
At UC Santa Barbara, researchers at the campus’s Center for BioEngineering (CBE) and the Department of Chemical Engineering have found that ionic liquids (IL), a known class of materials, can not only disrupt these biofilms, which increases the effectiveness of accompanying antibiotics, they can do so with virtually no irritation and inflammation of the already damaged tissue. Furthermore, their findings indicate that some ionic liquids, even without the use of antibiotics, are often capable of neutralizing pathogens. Their results are published in the Proceedings of the National Academy of the Sciences.
“The challenge was how to find a chemical, or a composition of chemicals, that are toxic to bacteria — they disrupt the biofilm and enhance the transport of antibiotic drugs — but do not kill the healthy mammalian cells or cause inflammation to the skin,” said CBE director Samir Mitragotri, senior author of the paper, who specializes in targeted drug delivery.
Ionic liquids are essentially salts — pairings of positively charged cations and negatively charged anions — that exist in liquid form under 100 degrees Celsius. These liquids have been known to scientists for over a century and are used in various applications, from solvents to additives to electrolytes.
For the study, Mitragotri, doctoral candidate Michael Zakrewsky and team synthesized several ILs, both common and novel, and assessed their performance on a biofilm on a wound model. They studied the liquids’ ability to break up the biofilm and enhance drug delivery, as well as for toxicity and antimicrobial activity. Of the liquids examined, one called choline-geranate emerged as a “multipurpose” IL that fit the researchers’ criteria most effectively.
Choline-geranate proved to be comparable to or even more effective than bleach against biofilms established by Salmonella enterica, a common cause of food poisoning, and Pseudomonas aeruginosa, a widespread bacteria that exists in many environments. The liquid also proved to be nonirritating and non-inflammatory, a rare quality for a material meant to enhance penetration through the skin. Additionally, even without an antibiotic, choline-geranate was effective against the target bacteria.
According to Zakrewsky, ILs are relatively easy to modify, so they can be used as multipurpose antimicrobials, as in the case of choline-geranate. Or they can be adjusted to the specific properties and microbes in the biofilm, even those that have become resistant to antibiotics.
“It’s really the fact that you have this one cation and one anion that you can tune individually within the pair that allows it to be very multifunctional,” said Zakrewsky, the lead author of the paper. “So we can choose one component that we know helps transport through the skin, and we can choose one component that we know disrupts biofilms.”
As microbial biofilm disruptors, ILs are good news for those who suffer chronic wounds, such as those related to diabetes, or suppressed immunities. Not only can ILs destroy existing biofilms, which can, over time, become impervious to treatment, they can do so without the inflammation that restricts the healing process. According to Scott Hammond, MD, executive director of UCSB’s Translational Medicine Research Laboratories, ILs also can be used to prevent infections from invasive procedures and to treat surfaces that can become the site of biofilms, such as implanted devices, surgical equipment, and even contact lenses, in addition to treating chronic ulcers.
“Chronic wounds are interesting to me because these patients come in two to three times a week for perpetuity, and we have completely hit a wall on what to do,” Hammond said. “There’s a whole spectrum of what we can do here,” he added, pointing out their use in pre-emptive practices and real-time profiling of patient and bacteria that can result in the optimization of therapy — and ultimately, less costly and more accessible healthcare.
The researchers credit their collaboration with partners at Los Alamos National Laboratory in New Mexico; Dixie State University in Utah and Northern Arizona University for the expertise that contributed to their findings.