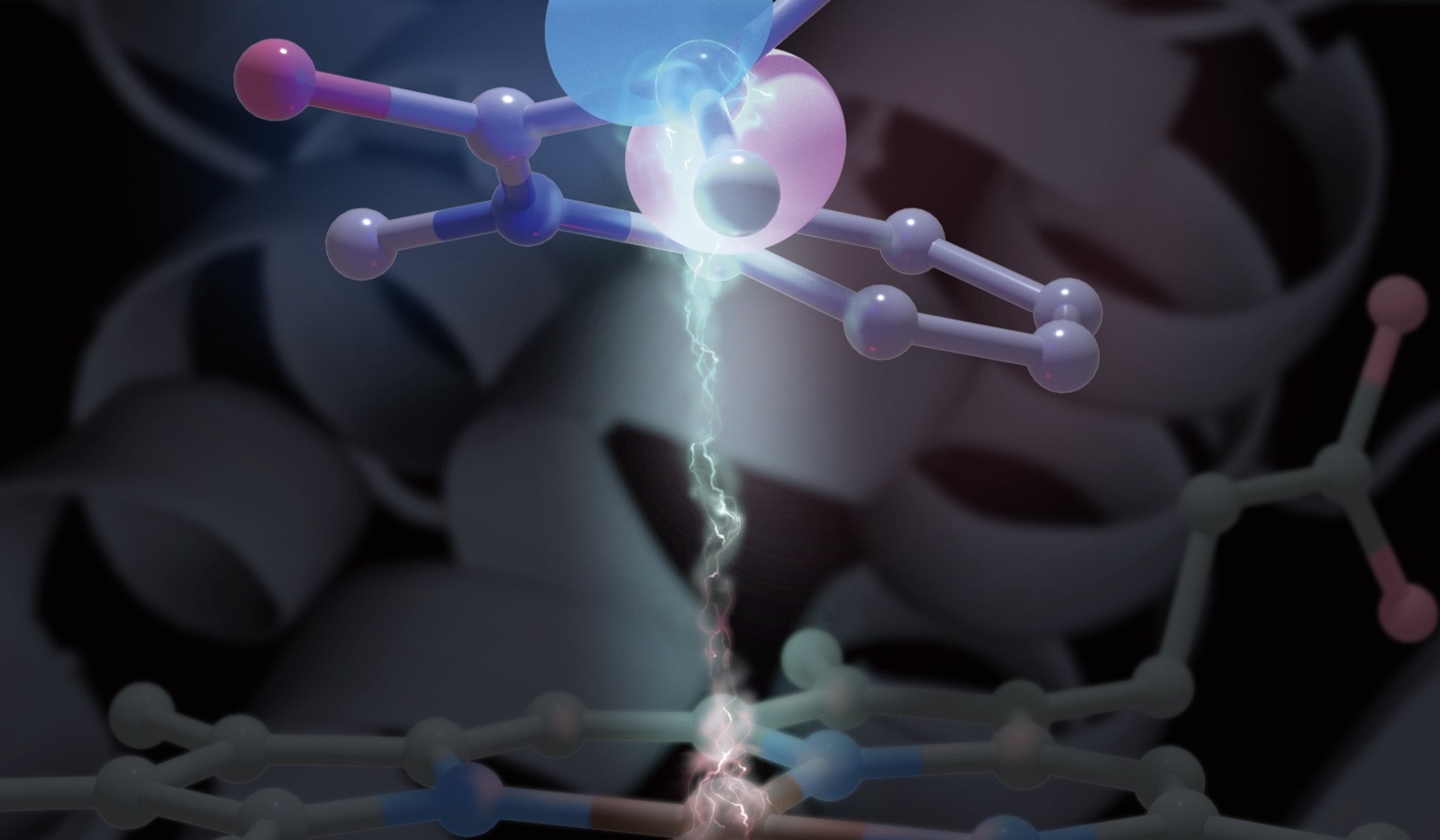
One of the central challenges for synthetic chemists is to impose control over free radicals. Highly reactive molecules with an unpaired electron, free radicals may be familiar to you; these are the type of molecules we take antioxidant supplements for, in an effort to tame oxidative stress.
In the world of synthetic chemistry, however, free radicals hold a lot of promise.
“Free radical chemistry is very useful for the synthesis of both bioactive small molecules and everyday polymers,” said UC Santa Barbara chemistry professor Yang Yang, an author of a paper on the matter that appears in Nature Catalysis. “However, imposing stereocontrol over free-radical mediated reactions has eluded the asymmetric catalysis community for decades. We’re trying to develop biocatalytic strategies to further push the boundaries of free radical chemistry.”
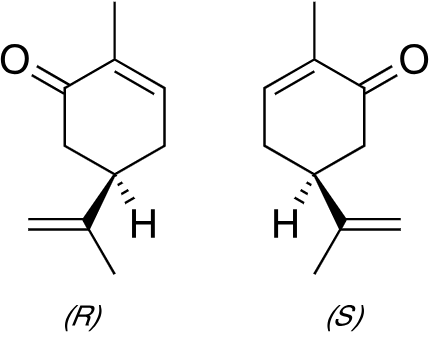
To fully unlock the synthetic potential of free radicals, Yang and colleagues focus on stereochemistry — also known as 3D chemistry, which focuses on the three-dimensional orientation of atoms and molecules. The stereochemistry of organic molecules has a significant impact on their properties. For example, (S)-carvone or “left-handed” carvone is the primary ingredient that accounts for the distinctive odor of mint. In contrast, (R)-carvone or “right-handed” carvone is found in caraway seeds and has a completely different smell. Thus, to precisely control the stereochemistry is a major goal of synthetic chemistry.
To achieve it, chemists turned to catalysts, substances that enable chemical reactions without themselves being consumed or transformed in the process, making them reusable.
Achieving this type of stereocontrol is no small feat. “In general, steering free radicals towards desired stereochemistry is very difficult,” Yang said. Free radicals, once formed, do not interact tightly with the catalyst. Additionally, these radicals are essentially free in another sense — they can quickly wander away from potential reactive sites.
But Yang and collaborators have a few tricks up their sleeves: metalloenzymes — naturally occurring proteins with a reactive metal center, able to generate and rein in those free radicals for selective transformations.
“Specifically in this paper we solve a problem in this field, which is how to control the stereoselective addition of a radical species to an aromatic compound,” Yang said. “The radical in this case is derived from racemic starting material.”

This is where the three-dimensional chemistry comes in. “Racemic” means that the material is composed of equal proportions of “left-handed” and “right-handed” (also known as “chiral”) molecules — asymmetric molecules that are composed of the same atoms, so are chemically identical, but are mirror images of each other. Like your hands, you can match them up reflection-wise, but you can’t superimpose them facing the same way. Which, under normal circumstances, would pose a problem for enzymes.
“Enzymes are widely perceived as very specific catalysts,” Yang said. “If the enzymes are so specific, which happens oftentimes in nature, then the enzyme can only recognize and convert one enantiomeric form of a chiral compound,” Yang said. “And oftentimes the enzyme doesn’t accept its mirror image.
“But in our work,” Yang continued, “we’ve engineered an enzyme that can accept both the left-handed and right-handed form of the starting material, and then convert these starting materials into the same major enantiomeric product with excellent selectivity.”
In their paper, the researchers used an iron-dependent enzyme to produce highly reactive radical species. Through directed evolution, they engineered a set of selective iron enzymes to produce either the left-handed or the right-handed product with excellent selectivity. Furthermore, with a third, “kinetic resolution” enzyme, the researchers can selectively convert the left-handed starting material, leaving the right-handed starting material untouched.
“So we have a toolbox of enzymes to allow for various types of stereocontrol for the radical functionalization of aromatic compounds,” Yang said. “And yet these enzymes only differ from each other by a few mutations.” Yang hopes this growing toolbox of biocatalysts will help others gain better control over their 3D chemistry, a classic problem that continues to face organic chemists.
“Our metalloenzymes provide a potentially general solution to control the selectivities of free radicals,” Yang said. “So these biocatalytic solutions we created will hopefully facilitate the synthesis and study of chiral compounds in academia and industry.”
Sonia Fernandez
Senior Science Writer
(805) 893-4765
sonia.fernandez@ucsb.edu




