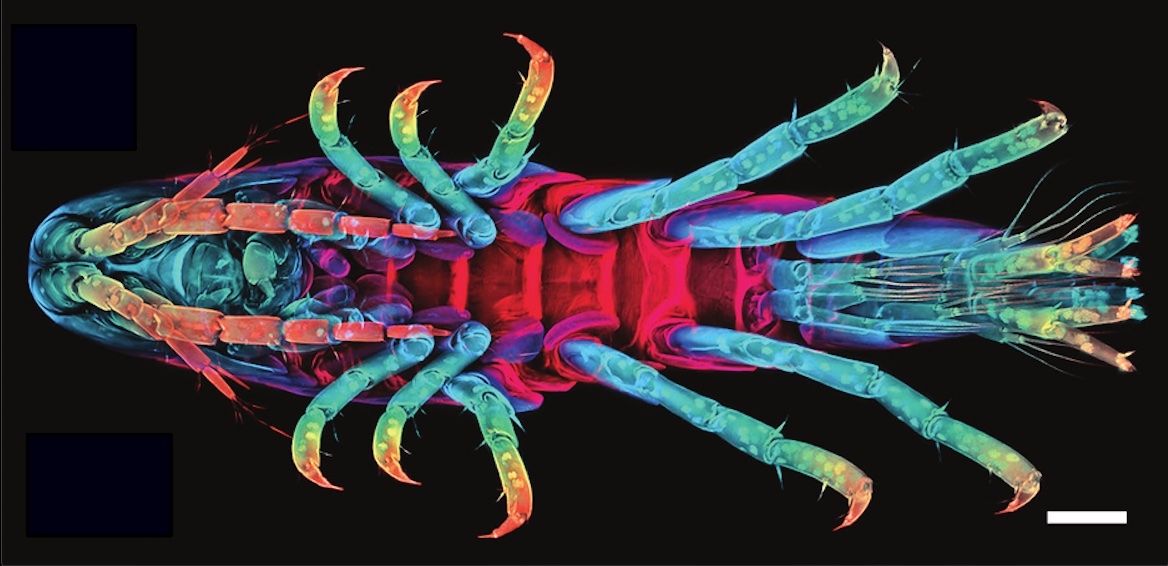
Consider the crustacean Parhyale hawaiensis, a tiny crustacean with some interesting attributes.
“It’s been called a ‘living Swiss army knife,’” said Dillon Cislo, the lead author of a study that appears in Nature Physics. “It has numerous different appendages and each one is uniquely specifiable by its size and shape. Furthermore, each one of these limbs has a very specific function.”
Their fascinating bodies and accessible growth conditions make these creatures a well-chosen model organism for developmental studies. But more than that, according to Cislo and UC Santa Barbara researchers Mark Bowick and Sebastian Streichan, their embryos are a window into the world of tissue morphogenesis, a field that seeks to understand how a mass of embryonic cells becomes the complex body parts of an adult organism. As a “direct developer,” or an organism that builds its adult form — albeit in miniature — as opposed to having a distinct larval form and undergoing metamorphosis, this crustacean is one to watch.
“You’re going from this set of randomly ordered cells into all of those crazy, highly articulated appendages in the adult structure,” said Cislo, a postdoctoral researcher at Rockefeller University who conducted research for this paper as a graduate student at UCSB under the guidance of theoretical physicists Bowick, Boris Schraiman, as well as Streichan, who specializes in the physics of living matter. Until recently, most observations of embryogenesis involved taking several embryos of a model organism — say, a fruit fly — at different stages of development and “fixing” them in order to freeze them in time. From there, scientists can make calculations and inferences as to the sequence of events that go into the development of their bodies. But what has been less easy to observe is how the young cells find their places and positions at all.
Finding out how it all works together is a hot topic in biology. But it also falls into the realm of active matter physics, a field that is interested in the collective behavior of systems of multiple independent “agents” locally consuming energy. Examples of active matter are diverse, from murmurations of starlings to bacterial colonies to crowds of people. Active matter can also encompass non-biological situations where unit components are out of equilibrium, such as robot swarms.
Order from Disorder
When embryonic cells divide, they do so in opposite directions along an axis, and then those daughter cells divide in opposite directions along their axes, and so on, though there is no reason why the division axis of a daughter must depend on the division axis of the parent. Which seems like it would complicate things for tissues whose structures and functions depend on the organization and orientation of their unit cells.
To see how P. hawaiensis’ cells tackled the disorder that could be presented by their proliferation, the researchers followed the development of an embryo, three days after fertilization.
“It looks like a thin layer of cells on top of a spherical yolk,” Cislo said. To better observe the process, they computationally flattened that curved set of cells into a plane “in a way that respected the three-dimensional geometry of the actual physical configuration,” the paper explains, and tracked these cells as they divided and moved around, in the first-ever dynamic analysis of this particular stage of P. hawaiensis’ early development.
Twelve hours after the observation start time, the growing population of cells had not only slightly more than doubled, they had arranged themselves into a grid, whose rows would correspond to the segments of the adult body. From there, the monolayer of cells, which roughly corresponds to the area of the belly of the crustacean, undergoes waves of cell division, starting from the midline and spreading laterally, dividing along the axis from the head to the tail of the animal-to-be.
The divisions weren’t random, Cislo said. That is, rather than merely becoming a bigger mass of seemingly unordered cells, these cells would divide, then some daughters would reorient themselves by as much as 90 degrees before dividing again in order to maintain their alignment with the head-tail axis.
“As it undergoes its division choreography, you begin to see new rows inserted between rows, pushing the rows above and below apart,” he said. “And this is very wild, because in a non-living physical system this is a very energetically expensive operation.” In metals and crystals, this mechanism of reorganization would require the material to be heated to thousands of degrees in order to become feasible, Cislo said, “but here the shrimp is doing it at room temperature.” To the best of the researchers’ knowledge, the general axis of the cell division most likely has to do with a biological signal yet to be uncovered.
Though fragile and in some cases energetically expensive, fourfold orientation in the case of early stages of the crustacean’s development is vital, according to the researchers.
“There are some ideas about how to interpret these results,” Streichan said. “The basic line of thought involves the orientation of animal limbs. Like our hands or legs, these limbs have clear orientations… and as the body consists of multiple such limbs, proper body function requires a coordination of the orientations of these limbs.
“Imagine your left hand was rotated with respect to your right hand, say 180 degrees swapping the back of your hand and the palm,” he added. “Daily tasks would become quite challenging!”
Shaking Things Up
One thing that’s important to remember is that this organization exists in a structured fluid state — not quite a fluid and not quite a solid, said Bowick. “From the physics point of view, the phase has the same form as a superfluid,” he explained.
It turns out for all the order generated by the gridlike organization of the cells, the potential for disorder presented by the fluid state and the cell divisions is crucial for the flexibility needed for a biological system, Bowick added. “The cells are not just dividing, they’re clearly exerting forces on each other as they do so,” he said.
The researchers found that the cells, each with its own little motor and its own “clock” for autonomous division, created a certain amount of “noise”— variations and fluctuations — throughout the early cell proliferation stage and in a subsequent stage where cells continued to divide but the tissue itself was also elongating. This noise may at first seem counterproductive to forming a complex body with so many different appendages, but, according to the researchers, the noise itself is necessary for a robust process. Utilizing its fourfold orientation, the system occupies a “Goldilocks zone” between order and disorder: enough order to begin to build the creature, but still open-ended enough to absorb slight discrepancies in the process.
Through a series of simulations, they found that despite variations in timing, or in concentration of divisions (to a certain extent), or the presence of cells that didn’t reorient themselves during proliferation, it was still possible to ultimately arrive at the same end result.
“The takeaway is that biology doesn’t really have to control things terribly tightly to achieve the desired result,” Cislo said — a finding that only a dynamic analysis could generate.
Bowick agrees. “Imagine that you want a system to reach some ordered state; if you’re completely static, you’d never find it,” he said. “But if you shake up the system, you might allow it to finally settle into a nice ordered state. And what seems to be going on here is that the cell divisions are shaking up the system, allowing it to finally settle into a subtle ordered state.”
This study provides a fascinating peek into a rarely seen facet of developmental biology, one that operates along a geometric organizational principle, as seen by its fourfold orientation.
“The fruit fly, which is the hydrogen atom of developmental biology, organizes the segments of its body plan via a cascade of biochemical signals,” Cislo explained. “This is something totally different.”
“What is cool about Dillon’s work is that the orientational order is found at the level of cell position, marking a mechanically observable ordered state,” Streichan said. In contrast to the development of other animals whose embryonic cells rely on chemical signals for orientation, in P. hawaiensis the grid patterning is a mechanical event that spans two regions — one close to and one farther away from the head, allowing both regions to agree on the positions of their cells. The grid also guarantees the locations and orientations of the cells that become the limbs even before they develop.
In many ways, Dillon’s project has provided yet another example that biology finds ways of leveraging physics for its purposes,” Streichan said.
“There could also be lessons for materials science,” added Bowick. “If you want to build interesting materials, you may want to take lessons from biology and drive some of these materials systems out of equilibrium, and make wonderful structures this way.”
Sonia Fernandez
Senior Science Writer
(805) 893-4765
sonia.fernandez@ucsb.edu



