The Multitudes Within Us

A romantic will say you carry your mother in your heart. Amy Boddy will say you carry her cells in your body. And now she’s going to lead an international team of scientists who intend to unlock the secrets of microchimerism (MC) — when cells from two genetically different organisms exist in one individual.
Thanks to a $5.4 million grant from the John Templeton Foundation, Boddy and her team will use cutting-edge technology to investigate how MC affects a host’s physiology — one of the key questions about the little-understood phenomenon.
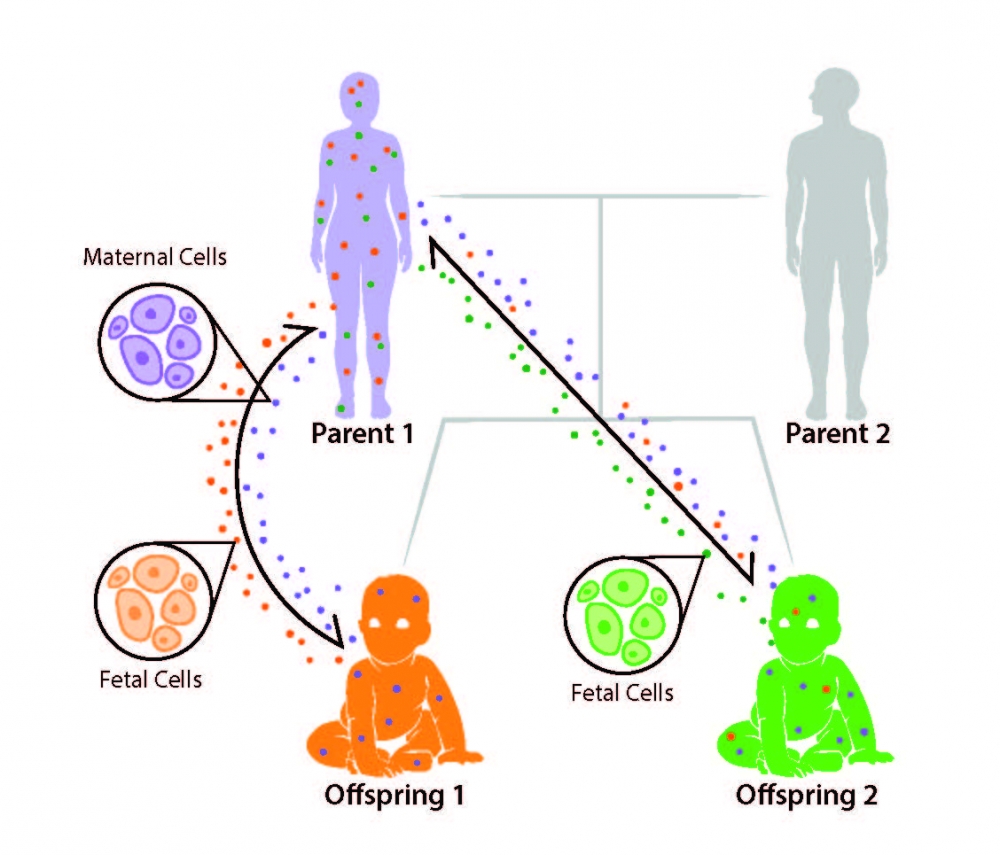
In humans this cellular exchange occurs between mother and fetus during pregnancy, and microchimeric cells are presumed to endure for the lifetime of the host. That makes us all chimeras who might carry cells derived directly from our ancestors and offspring.
The open questions about MC, however, are legion. It’s generally thought it has both beneficial and negative effects. MC cells can differentiate into almost any cell type in the host and implant in almost any tissue type, including the host’s brain.
MC has been proposed to play a role in maternal wound healing, but may also be associated with pregnancy complications, such as pre-eclampsia and spontaneous abortion, as well as cancer and auto-immune diseases. MC could also play a role in providing immunological protection for the developing fetus, but it’s also been associated with offspring autoimmune disorders.
“MC has puzzled researchers for over four decades, with many paradoxical implications for human health and disease,” said Boddy, a UC Santa Barbara professor of anthropology whose research focuses on evolution and human health. “Results from our work have the potential to create a paradigm shift in how we understand mother-infant biology.
“From an evolutionary perspective,” she continued, “we propose the transfer of maternal (and possibly deeper generational) cells is important for offspring reproductive success. The co-evolution of microchimerism and its effects on maternal and offspring immune function has major implications for the evolution of placentation, immune tolerance during pregnancy and sex-biased diseases, including cancer and autoimmune disease.”
Sarah McClure, an associate professor and chair of the Department of Anthropology, noted the grant will fund important new work on uncovering, characterizing and understanding biological mechanisms that impact human health and disease.
“This research is an international collaboration and a wonderful recognition of Dr. Boddy’s contributions over the last several years that have been integral in changing our perspective on these issues,” McClure said. “As part of this project she will also be working with scholars and medical practitioners in the community, including Cottage Hospital and various OBGYN clinics.”
Boddy’s interdisciplinary team — experts in immunology, evolutionary biology, genomics, analytical chemistry and cell biology — came together unconventionally: by Zoom and over email during last year’s COVID lockdown.
“We started as a small group just thinking about some really neat aspects of microchimerism that have evolutionary applications,” she said, “which led to more questions and more invitations of individuals with specific expertise in the technology that could answer that question. I am most excited about the new technology and the interdisciplinary perspective we will be using to study microchimerism. This allows us to have a really exciting ‘toolbox’ of approaches to study microchimerism.”
Most previous MC research, Boddy said, has been epidemiological in nature and attempted to quantify one side of the maternal-fetal “crosstalk.” But to fully understand the complexities of MC, she added, the team will need to integrate and develop novel technologies and approaches to characterize the cross-talk between mother and offspring — because each individual dyad can be different.
“Many of the open questions in MC can be addressed by first understanding the ‘where and how’ of how MC tracks in the host body,” Boddy explained. “Our overarching ambition is to create a ‘microchiome atlas’ to understand the distribution of microchimeric cells in mothers and offspring across multiple generations and define the mechanisms of MC cell transfer and tolerance. Tackling this is only possible with a cross-disciplinary team.”
That team, with scientists from the U.S., Austria, Germany and Japan, is expected to be an important contributor to the growing field of MC research, Boddy said.
“The mother-child dyad is one of the deepest inter-organismal connections in the animal kingdom,” she said. “This cellular exchange solidifies a shared biological connection which lasts a lifetime — and has the potential to transfer through multiple generations (including grandmother cells) — and I just think that kind of biological phenomenon is so amazing! We contain genetic multitudes.”