Productive Plasmons

A good discovery can bear fruits for decades, shaping an individual’s entire career. Such was the fortune of UC Santa Barbara’s Martin Moskovits. It was back in 1978 that he first proposed the plasmon theory for surface-enhanced Raman scattering (SERS), a peculiar effect in spectroscopy whereby rough surfaces can increase the signal a million-fold.
Four decades later, the distinguished professor is still involved in the debate and discussion about SERS. In a new paper, published in the journal Matter, Moskovits and his coauthors offer an up-to-date account of current research on plasmon-mediated chemical reactions and discuss the challenges and opportunities scientists face in understanding the phenomena and developing them further.
“The excitement about this effect, which I thought would blow over after a year or so, just never stopped,” Moskovits said. “If anything, its appeal has only grown.”
Spectroscopy is an analytical method that, among other things, can determine the chemical nature of materials by the manner in which they interact with visible light and other forms of electromagnetic radiation. Spectroscopic techniques can reveal a great deal about a material’s structure and composition. For instance, the bonds between atoms in a molecule are flexible, “as if they have springs that hold them together. “Measuring the quantity of light they emit or absorb can often serve to identify the molecules,” explained Moskovits.
In a technique called Raman spectroscopy, researchers can measure the vibrational patterns — as revealed by the light the molecules emit when tickled by a laser — to learn the molecule’s composition and geometry.
Back in 1977, researchers at Northwestern University noticed that certain rough, metallic surfaces yield a signal a million-fold larger than smooth ones. At that point no one understood how or why this happened, though a number of theories were proposed.
Around this time, Moskovits was investigating the properties of clusters: collections of bound atoms or compounds between the size of a molecule and a bulk solid. While pursuing these topics, he discovered that electrons behave differently on rough surfaces than on smooth ones.
“By making a little feature on the surface you open up new ways for the electrons to oscillate and absorb light,” he said. For instance, the electrons trapped in little surface bumps have unique resonances. These light-driven oscillations have been dubbed plasmons.
Moskovits suggested that SERS could be explained in terms of plasmons. Effectively, the small metal particles act as antennas, enhancing the light emissions of the molecules resting on them.
Subsequent research has mostly borne out this theory. And, as the phenomenon attracted more interested minds, “plasmons, as a field of study, blossomed into a field of its own,” Moskovits added.
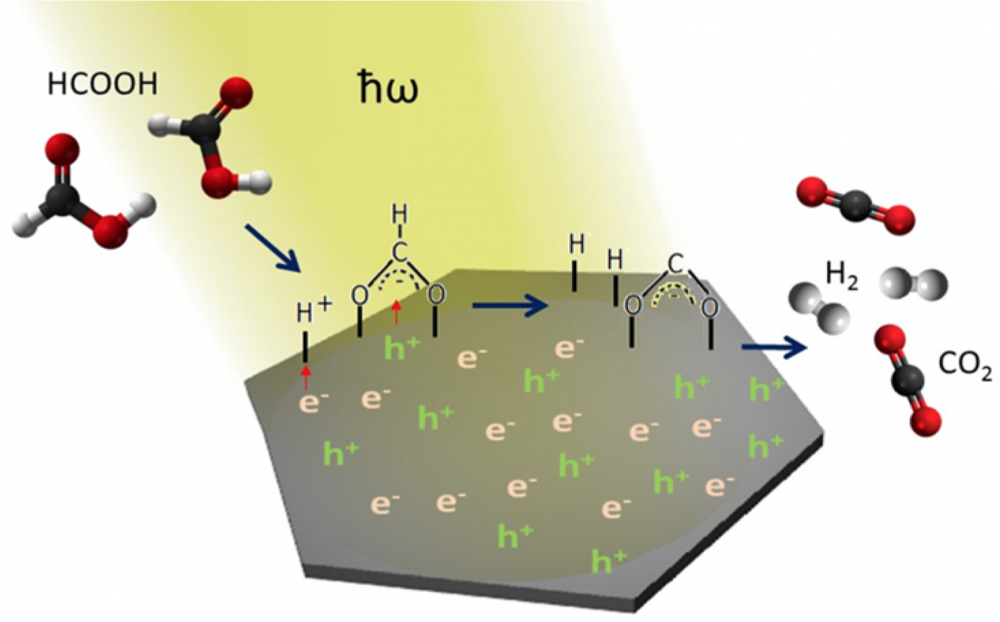
Soon, scientists realized that SERS held promise beyond just chemical analysis. The excited electrons, enhanced electric fields and localized heating from SERS could all be put to work carrying out chemical reactions. Scientists could now increase the efficiency of all sorts of light-mediated reactions by large factors. This elevated surface-enhanced Raman from an intriguing trick to a bona fide spectroscopic technique.
The field of plasmon-mediated chemical reactions faces two remaining challenges, Moskovits states in the recent article: untangling the multiple processes that contribute to these reactions as well as improving system design and fabrication.
Researchers are still working to disentangle the multiple processes underlying SERS. For instance, scientists have yet to devise an account that distinguishes the contributions of photothermal and photoelectric effects. Progress in the field depends on sorting out these details, the paper notes.
On the pragmatic end, most demonstrations have focused on improving reaction rates. However, the authors point out that reactivity of the plasmonic material seems to be the real limiting factor right now.
One of the areas where plasmon-mediated chemistry shows promise is in making more effective use of solar power, particularly by taking advantage of sunlight at levels currently considered too low to be useful. For instance, Moskovits’ former postdoctoral research assistant Syed Mubeen, now a faculty member at University of Iowa, constructed a device that can split water into hydrogen and oxygen simply using ambient sunlight. This technology could lead to more cost-effective methods for producing hydrogen for energy.
“From my perspective, this is really an old story,” Moskovits said, “but it just seems to persist.
“Why? Because people think up new ways of making use of it.”



