Tracing the Roots of Schizophrenia
Scientific consensus holds that most major mental disorders, such as schizophrenia, are genetically rooted diseases of synapses, the connections between neurons in the brain. Now research has demonstrated how a rare mutation in a suspect gene corrupts the on-off switches of dozens of other genes underlying these connections.
The study appears online in the current issue of the journal Nature. Employing a disease-in-dish technology called induced pluripotent stem cells (iPSCs), the team of investigators, including UC Santa Barbara researchers, studied iPSCs from four members of an American family affected by genetically linked schizophrenia and related mental disorders.
Decades ago, researchers traced a high prevalence of schizophrenia and other major mental disorders — which often overlap genetically — in a Scottish clan to mutations in the gene DISC1 (Disrupted In Schizophrenia 1). However, until now, most of what is known about the cellular effects of such DISC1 mutations has come from rodent brain studies.
“This new study’s design was the first of its kind to examine multiple affected and healthy members of the same family,” said co-author Matthew Lalli, a Ph.D. candidate working in the Kosik Molecular and Cellular Neurobiology Lab, which conducted the RNA sequencing portion of the study. “Our results showed a clean, close resemblance of the neurons cultured in a dish to some biochemical features of the disease.”
The research team collected skin cells from a mother and daughter who have neither the variation nor mental illness as well as the father, who has the variation and severe depression, and another daughter, who carries the variation and has schizophrenia. For comparison, they also collected samples from an unrelated healthy person.
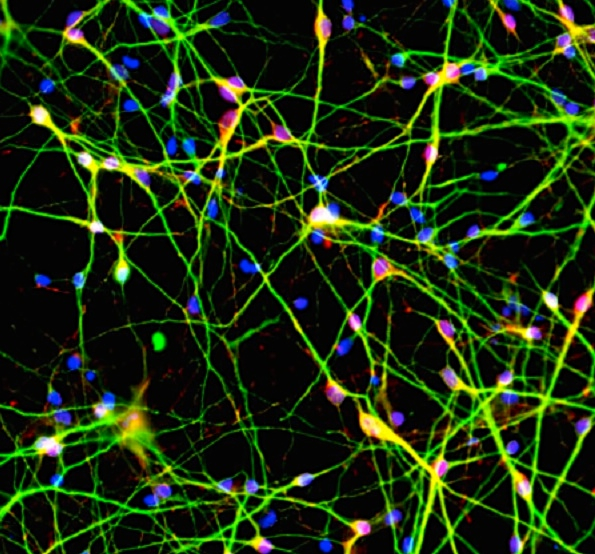
Lead author Zhexing Wen, a postdoctoral fellow at the Institute for Cell Engineering at Johns Hopkins University School of Medicine, coaxed the skin cells to form five lines of stem cells and to mature into very pure populations of synapse-forming neurons. UC Santa Barbara scientists observed how these patient-derived neurons developed and interacted in a petri dish in order to determine the effects of the genetic variation on young brain cells.
“One unexpected result was that many of the dysregulated genes were interaction partners with DISC1, the gene that causes disease,” said. Lalli. “The other finding is that a lot of the disrupted genes were already known to contribute to various mental disorders.”
The researchers found that the iPSC-induced neurons — of a type found in frontal brain areas implicated in psychosis — expressed 80 percent less of the protein made by the DISC1 gene in family members with the mutation compared to members without the mutation. In a cascade effect, the gene products that interact with DISC1 were also reduced. These mutant neurons showed deficient cellular machinery for communicating with other neurons at synapses.
“We didn’t know that the disease would manifest so clearly at synaptic junctures and be expressed so clearly in a dish,” said Lalli. “But this work confirmed our hypothesis that schizophrenia is at least partially a disease at the synapse.”
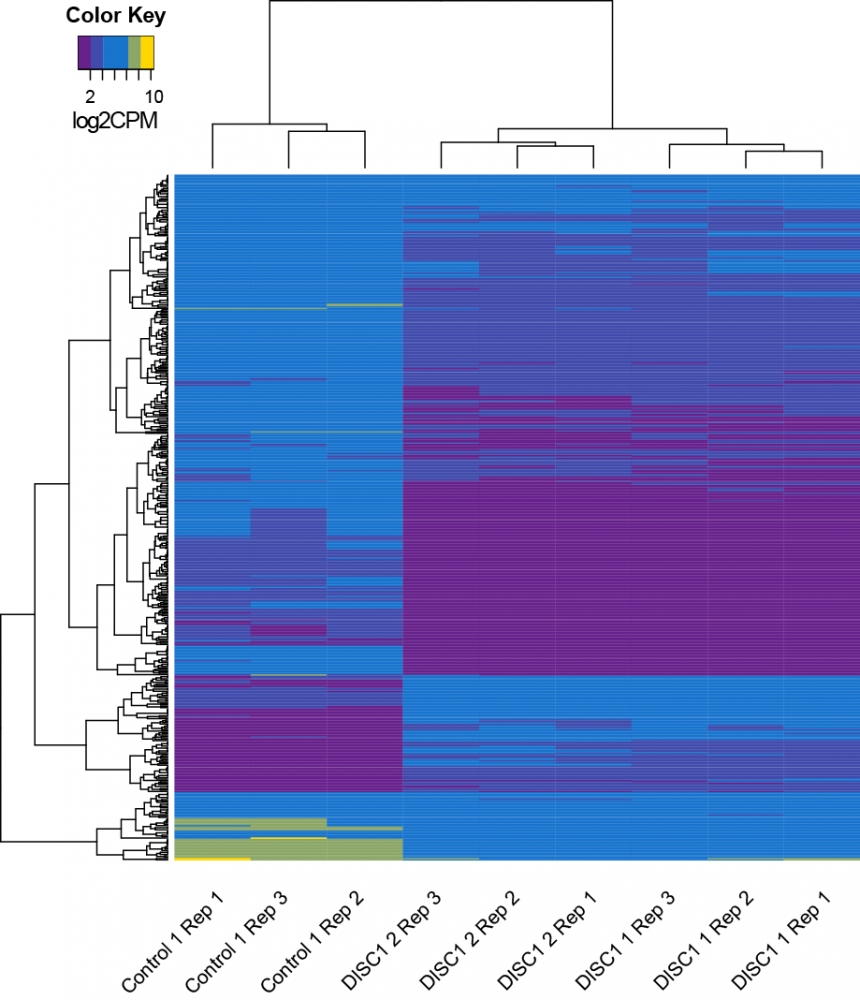
To find out how DISC1 acts on synapses, the researchers also compared the activity levels of genes in the healthy neurons to those with the variation. To their surprise, the activities of more than 100 genes were different. According to the scientists, this is the first indication that DISC1 regulates the activity of a large number of genes, many of which are related to synapses.
“The fact that the single mutation could be corrected in the disease line or introduced to the healthy line converting it to a disease line really implicated this gene as being causal of the disease,” Lalli explained.
“The ability to re-reate features of schizophrenia in neurons produced from patient skin fibroblasts brings us one step closer to developing treatments because this model system is highly suitable for screening drugs that could repair the biochemical defects,” added co-author Kenneth S. Kosik, the Harriman Professor of Neuroscience in the Department of Molecular, Cellular and Developmental Biology and co-director of campus’s Neuroscience Research Institute.
The work was supported by the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, the One Mind Institute, the Simons Foundation Autism Research Initiative, the Maryland Stem Cell Research Fund, the Brain and Behavior Research Foundation and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation.