Predicting Neural Toxicity

Stem cells have a multitude of uses, not the least of which is to create tissue models that reflect human physiology. Such stem cell-derived models have enormous potential in research and application.
One possible use, developed by a team of scientists from UC Santa Barbara, the University of Wisconsin-Madison and the Morgridge Institute for Research in Madison, involves reducing the number of drug failures in clinical trials and offering a cost-effective approach for assessing chemical safety.
The researchers have developed a screening system for predicting developmental neurotoxicity — damage caused to nervous tissue by toxic substances — using stem cells to model features of the developing human brain. The findings appear today in the Proceedings of the National Academy of Sciences.
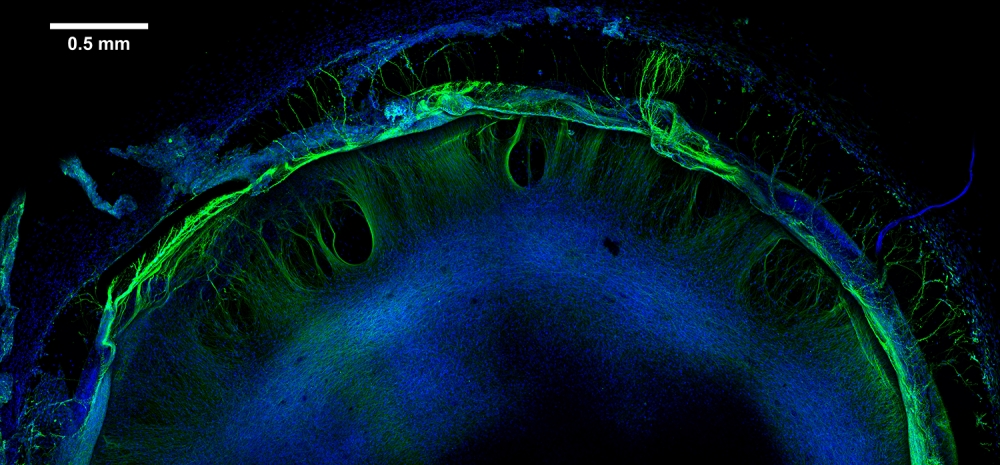
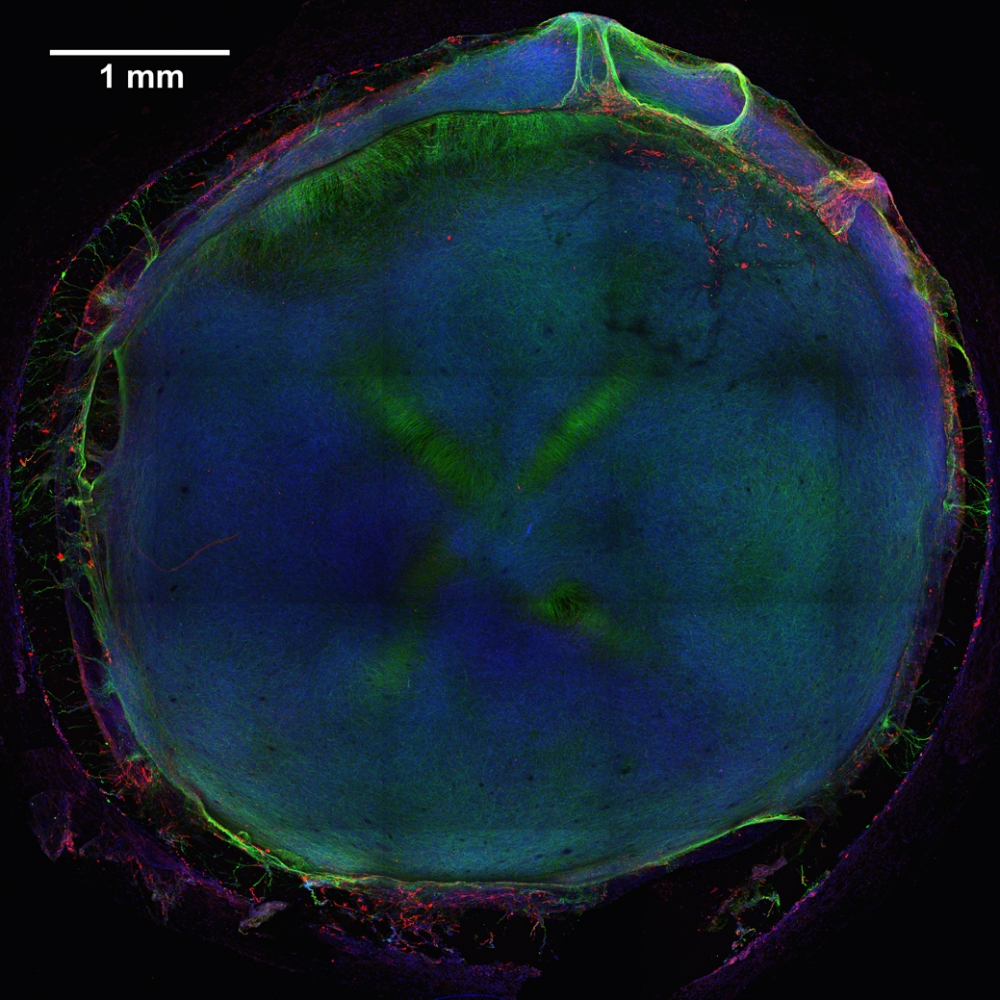
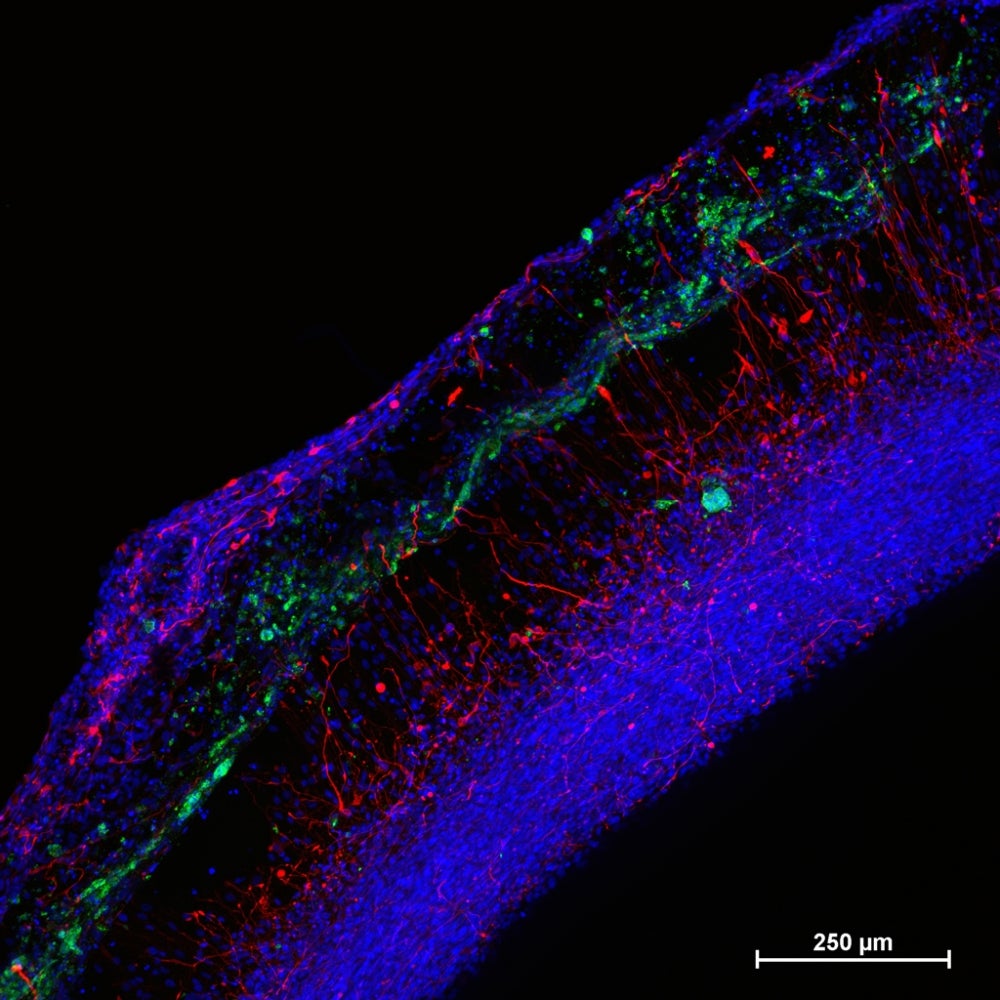
To begin, the team produced model human neural tissue by culturing stem cell-derived neural progenitor cells, vascular cells and microglia on engineered hydrogels. These precursor cells self-assembled into three-dimensional neural tissue constructs with features that resemble the developing human brain. Such tissues are often referred to as “organoids.”
“We looked at the entire portfolio of gene expression in these constructs and how they respond to chemicals at different stages in development,” explained James Thomson, a professor in the Department of Molecular, Cellular, and Developmental Biology, where he serves as co-director of the Center for Stem Cell Biology and Engineering. “Then we used a machine-learning algorithm to tell us whether or not a chemical or drug has a toxic profile or fingerprint.”
RNA-sequencing data was collected from neural tissue constructs individually exposed to 60 different “training” chemicals — both safe compounds and known toxins. A remarkably accurate algorithm was used to build a predictive model from these results. After training with known chemicals using duplicate samples and two time points (240 neural constructs in total), the model correctly classified nine out of 10 additional chemicals in a blinded trial.
“Several things about this project surprised us,” said Michael Schwartz, an assistant scientist in biomedical engineering at UW-Madison and co-lead author of the study with Zhonggang Hou of the Morgridge Institute (now a researcher at Harvard University). “In the beginning, we weren’t expecting the kind of complex neural tissues that were ultimately developed.”
Schwartz says that this new screening method offers a valuable bridge between testing a single layer of cells in a dish and testing on animals. “These model neural tissues capture a lot more of the complexity than you would find in a monolayer of cells,” he said. “They also mimic human physiology and should be more relevant for predicting toxicity than animal models. The fact that we could apply a machine-learning model to achieve 90 percent accuracy this early in the process is fantastic.”
Thomson, also director of regenerative biology at Morgridge and a professor at the University of Wisconsin School of Medicine and Public Health, noted that the project has potential to improve drug testing, but with more than 100,000 mostly untested chemical compounds used in commerce, the impact could be even greater for screening chemicals.
“Current toxicity screening tests use multigenerational rat studies and cost about $1 million to test one chemical,” he said. “So we need a really high-throughput way to test these compounds, figure out which ones may be the bad actors and then focus on those with more traditional methods.”
According to Schwartz, the RNA sequencing data generated by this study will be beneficial to future studies by helping to identify potential toxic profiles or fingerprints. “These datasets provide valuable information about changes in gene expression that researchers can mine to better understand mechanisms that might be disrupted during human brain development,” he said.
The neural tissue constructs developed in this project are the first to incorporate vascular and microglial components into a 3-D model of brain development derived from human pluripotent stem cells. The synthetic material used to help the tissues grow was a key part of the early success of this work.
“These hydrogels are minimally complex in that they only present peptides that allow the cells to attach and degrade the matrix,” Schwartz explained. “The cells will do the rest of the work on their own; biology does a better job forming tissues than we do.”
Thomson noted that “if appropriately specified, precursor cells are brought together in the right environment, a degree of self-assembly, differentiation and maturation will occur.” The synthetic materials used to culture the cells were key to achieving the consistency needed to successfully screen so many samples.
The National Institutes of Health funded this project along with 10 other universities in the Tissue Chip for Drug Screening program. The Environmental Protection Agency provided the list of well-characterized toxic and nontoxic chemicals used in the study.
Other co-authors include Nicholas Propson, Jue Zhang, Peng Jiang, Bao Kim Nguyen, Ron Stewart and Yu Wang of the Morgridge Institute; Collin Engstrom of biostatistics and medical informatics and computer science and William Daly of biomedical engineering at UW-Madison; and Vitor Santos Costa of the University of Porto in Portugal.