Unraveling a Biomedical Mystery

With no cure, no approved treatment and no known means of slowing its progression, polycystic kidney disease is a biomedical cold case that affects more than 600,000 people in the U.S. alone — and some 12 million worldwide.
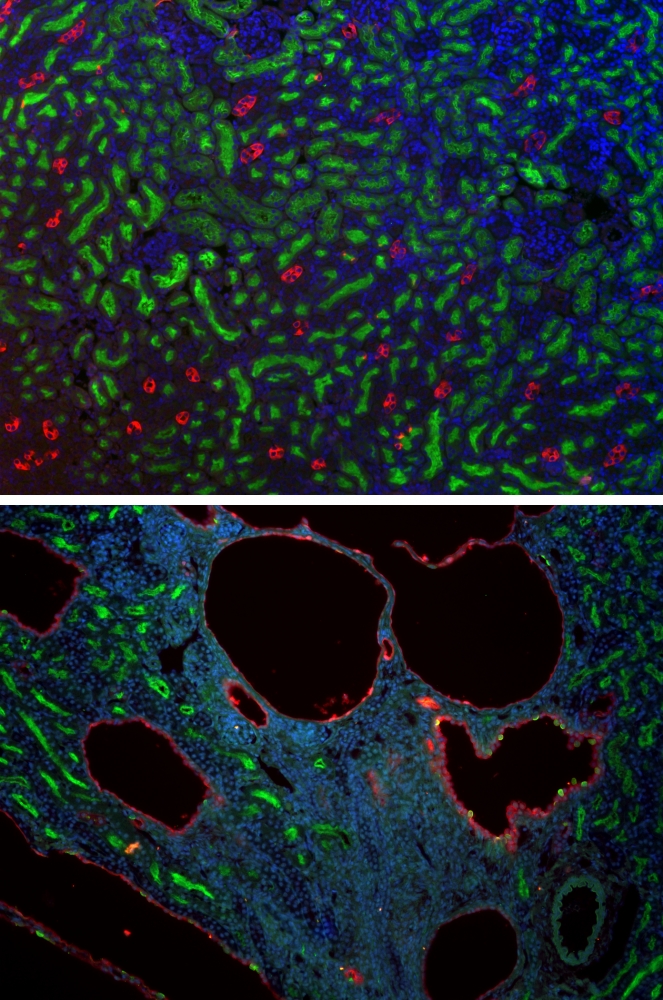
Hoping to crack that case with science, UC Santa Barbara biologist Thomas Weimbs is working to develop a new therapeutic approach by targeting the mechanism that causes the cysts to grow. A new gift to his lab is providing a big boost to that effort.
After a competitive selection process, the Lillian Goldman Charitable Trust of New York has awarded $600,000 to Weimbs to support his team’s continued work on autosomal-dominant polycystic kidney disease (ADPKD or PKD). Weimbs is one of the world’s top experts in the disease, a potentially fatal genetic condition that leads to renal failure and causes major cardiovascular complications.
“We are thrilled and grateful to have received funding from the Lillian Goldman Charitable Trust, which will greatly help to accelerate the progress of our research,” said Weimbs, a professor of molecular, cellular and developmental biology. “My lab is very focused on understanding the mechanisms that are most critical for the development of the thousands of cysts that eventually destroy both kidneys in PKD patients. We are driven by the motivation to develop a feasible therapy to at least slow disease progression and are working on several highly promising approaches in parallel. Philanthropic, targeted funding such as this can make a huge difference in speeding up the progress of research.”
Considered the most common life-threatening, monogenic inherited disease, PKD is a leading cause of kidney failure. With no viable treatment available, most patients eventually require a renal transplant or lifelong dialysis for survival. Research in Weimbs’ lab is aimed at understanding the molecular mechanisms that lead to renal cyst growth and disease progression — and at identifying and testing new strategies for therapy.
“This disease is slowly progressive, over decades, essentially, the kidneys grow bigger and bigger and become more cystic, and destroy the normal kidney tissue,” Weimbs said. “Usually when patients are in their fifties or so, on average, the tissue has been so destroyed that the kidneys stop functioning.”
More common than cystic fibrosis, muscular dystrophy, Down syndrome and sickle cell anemia combined, PKD affects one in 500 people worldwide. As it progresses, cysts grow and multiply such that a kidney can expand from the size of a human fist, which is normal, to be as large, or larger than, a football, and weigh nearly 40 pounds each.
Parents with the dominant form of the disease have a 50 percent chance of passing it on to each of their children.
Over the years there have been several clinical trials to test the efficacy of various drugs for treatment of PKD, Weimbs said, but most showed little, if any, benefit.
“Patients have high hopes that there may be a successful drug treatment out there, and I think they are correct but this has proven to be a hard nut to crack,” said Weimbs, who has been working on PKD for more than a dozen years. “It’s a genetic disease and the same gene mutation passes from parent to child. But strangely, the rate of progression is widely variable. While the parent may progress to end-stage renal failure at, say, 60, their child might have a faster progression and reach end stage 10 or 20 years earlier, or the other way around. It’s inconsistent.
“It has been speculated there could be other genetic factors at play, or even environmental factors,” Weimbs added. “We are exploring those possibilities and are particularly excited about identifying an environmental factor because this would potentially allow much easier intervention.”



