Bone Deep

Many diseases of the body are tied to changes in the structure and composition of the tissue, but often, such as with osteoporosis, the diagnosis isn’t made until after significant damage has been done.
In an effort to improve early diagnostics and give physicians and patients a head start in fighting and even preventing the progression of the debilitating bone disease, UC Santa Barbara researchers Jean Carlson and Chantal Nguyen have partnered with scientists at local startup bioProtonics to develop and validate a method of measuring — at the micron level — structural changes in trabecular bone.
A novel technique that uses existing magnetic resonance imaging (MRI) technology, it could result not only in improved quality of life for the millions of people at risk for bone disease every year but also, potentially, in fewer trips to the hospital and less money spent on treating fractures due to porous and fragile bones. The UC Santa Barbara researchers are collaborating with Timothy James, Ph.D., bioProtonics’ chief executive officer, and Kristin James, Ph.D., vice president of research and development.
“The goal is ultimately to be able to extract markers of bone health that are determined from the architecture of trabecular bone itself,” said Nguyen, a graduate student in physicist Carlson’s research group.
Their study, “Novel magnetic resonance technique for characterizing mesoscale structure of trabecular bone,” appears in the journal Royal Society Open Science.
Trabecular bone is the “spongy,” relatively more flexible bone tissue found in various places in the body, such as the ends of long bones, shoulder blades and skull, and in the vertebra and hips.
“You need bone to be lightweight; you need it to be able to rebuild itself all the time,” said Carlson, noting that the spaces in trabecular bone’s weblike structure make room for blood vessels, nerves, and bone marrow. While the dense, compact structure of cortical bone provides strength and form to the body, the open structure of trabecular bone also serves to dampen stresses to the joints.
“When you look at trabecular bone, you see all of this internal structure, so our work has been an effort to try to understand how those structural elements are important, how you could measure them, and how they contribute to the strength of the material,” explained Carlson, who studies the physics of complex multiscale systems, and how various tiny structural elements contribute to the behavior and characteristics of larger systems.
While technology already exists to test bone density, Nguyen said, it has limitations. “There have been studies that show that bone mineral density isn’t that well correlated with the strength of bone and with fracture risk,” she said. To assess bone health, it’s important to examine the microstructure of the interconnected struts that make up trabecular bone, beyond bone mineral density.
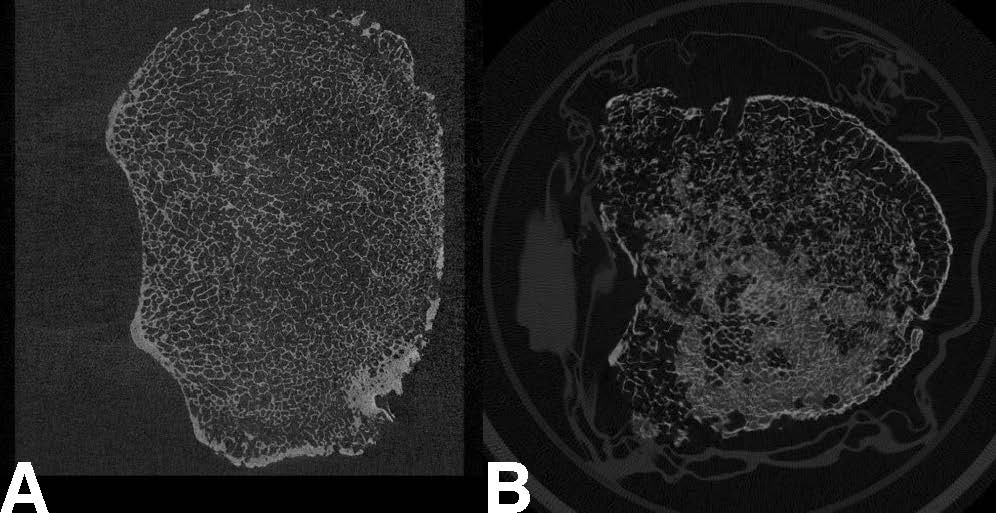
“The problem is if you want to image the vertebrae at high enough resolution to resolve the microstructure, you would need to use micro-CT, which requires a lot of radiation,” Nguyen said. “So this isn't really achievable currently in humans.” Conventional MRIs, meanwhile, do not use ionizing radiation, but even the slightest motion — a breath, a heartbeat — can negatively impact the desired image resolution.
In conjunction with diagnostics company bioProtonics LLC, simulating their µTexture MRI imaging technology (“µ” indicates “micron” — about 1/50th the diameter of a fine human hair), the Carlson research group devised a method to track the pattern of composition in trabecular bone.
Simulating measurements taken with the µTexture MRI technology, the researchers opted to take repeated samples of the frequency of certain patterns representative of trabecular bone architecture — as opposed to using all the values typically sampled in an instance of MRI measurement — as they move along a target bone. In this case: a vertebra from a cadaver.
“Instead of making a comprehensive image, by sampling all locations and all frequencies across an entire specimen, we’re sampling certain values in specific locations that are relevant to predicting the micro-structure,” Nguyen said. By taking relatively few but more relevant measurements, they can assess the texture of the bone without acquiring all the data needed to produce a full image, thus getting around the issue of motion. Using measurements of both healthy and osteoporotic bone and adapting previous work developed for the measurement of jaw bone composition, they developed a ratio metric that can indicate the relative texture of potentially osteoporotic bone in its earliest stages.
One of the challenges in measuring the relative porosity in trabecular bone is its irregular structure, and the fact that bone density changes from person to person.
“Even within an individual, the structural measurements related to bone textures can be highly variable,” Carlson said. Generally speaking, however, there are noticeable differences in structural composition between younger and older people. “The hope is that we can make enough point measurements and sample enough locations to see the degradation that underlies the disease state,” she said.
While this project focuses specifically on bone composition, the technique could be extended to other conditions in which changes in tissue textures are an indication of disease, such as tumor formation and fibrosis.
“The µTexture MRI technology could be used to detect Hepatitis C and fatty liver disease,” Nguyen said, adding that the method could be adapted to also find cardiac conditions, tumor formation, as well as changes in neuronal architecture cause by Alzheimer’s and other neurodegenerative diseases.